Promote blood circulation, reduce the risk of coronary thrombosis causing thromboembolic angina.
Used for following circumstances:
People who are at risk of coronary thrombosis and have early manifestations of coronary heart disease such as shortness of breath on exertion, tachycardia, palpitations, insomnia, myocardial ischemia, history of embolism.
Myocardial Infarction Model induced by Isoproterenol in Rats and potential Cardiovascular Protective Effect of hard capsule
Effect of PRODUCT on relative heart weight
In the present study, significant increase was observed in relative heart weight in all groups administered ISO as compared to the normal control group (p < 0.001). Treatment with PRODUCT did prevent the ISO-induced increased relative heart weight but non-significantly in comparison with the negative control group administered solely with ISO (p > 0.05) (Table 1).
Effect of PRODUCT on blood pressure measurements
Administration of ISO (150 mg/kg BW) in ISO group showed a significant increase in systole blood pressure as compared with the normal control group (p < 0.05). There was also a dramatic increase in systole blood pressure of PRODUCT treated group and no significant change was observed when compare the results from treated groups with negative control group (p > 0.05). There is no notable change in diastole blood pressure and heart rate in all 4 groups (p > 0.05) (Table 1).
Effect of PRODUCT on ECG
At the beginning of the experiment, there was no abnormal sign on ECG in all 4 groups. On the 31st day ECG images have shown a totally reverse result between the normal control group and negative control group in the ratio of abnormal ECG: 100% ECG images in negative control group had pathologic changes, whereas none (0%) in the normal control group ECG images had such changes. The changes observed in ISO-induced groups ECG included: ST segment elevation, negative T waves and pathologic Q waves. In the PRODUCT treated groups: 63.6% and 40% ECG images of group 3 and 4, respectively, had abnormal changes (Figure 1). There was a statistical difference observed in group 4 compared to negative control group with p < 0.05.
Effect of PRODUCT on biochemical parameters
Rats administered with ISO (150 mg/kg, i.sc) showed a significant increase in all biochemical parameters: AST, ALT, LDH, CK, TnT (p < 0.001) as compared to the normal control group. Group 4 (treated PRODUCT 0.92 g/kg, orally) showed improvements in LDH and AST as compared to ISO-induced group (p < 0.01 & 0.05, respectively), lowered the ALT, CK and CK-MB levels in plasma but had non-significant change as same as group 3 (treated PRODUCT 0.31 g/kg, orally) (p > 0.05). Especially in TnT plasma levels, there was a dramatic decrease as compared PRODUCT treated group to negative control group (p < 0.001 in both comparisons) (Table 1).
Effect of PRODUCT on anti-oxidative enzyme catalase in heart tissue
Oxidative stress was observed in negative control group through the statistically significant decrease in catalase (CAT) concentration in heart tissue after the experiment as compared to the normal control group (p < 0.05). PRODUCT did improve the anti-oxidative capacity by increasing the heart CAT concentration. The heart CAT levels in both treated group were significantly higher than that of the negative control group with p < 0.01, and even higher than the normal control group but non-significantly (p > 0.05) (Table 1).
Effect of PRODUCT on eNOS concentration in plasma
In the present study, no considerable change was observed between the mean values of eNOS while comparing the normal control group with the negative control group (p > 0.05), the levels of eNOS were as follow: 2.49 ± 0.82, 2.42 ± 0.50 ng/mL respectively. Administered PRODUCT produced significantly increase in eNOS levels as compared to both negative control group (p < 0.01) and normal control group (p < 0.01). The greatest increase was from group 3 (ISO + PRODUCT 0.31 g/kg) with a mean value of 7.34 ± 2.08 ng/mL followed by group 4 (ISO + PRODUCT 0.92 g/kg) with 3.83 ± 1.15 ng/mL (Table 1).
Effect of PRODUCT on histopathological studies
Histopathological examination of the normal control group did show the clear integrity of myocardial cells with no leucocyte infiltration (LIF), inflammation, vascular congestion or necrosis. Administered ISO 150 mg/kg solely did cause the moderate-to-severe multifocal myocardial necrosis, also infiltration of leucocytes and vascular congestion at the same degree. Rats treated with PRODUCT in both group 3 and 4 exhibited mild to moderate necrosis with less LIF and vascular congestion (Figure 2).
Table 1. Effect of TD.HK01 on relative heart weight
| Group | Control | Negative control | TD.HK01
0.31 g/kg |
TD.HK01
0.92 g/kg |
| Effect of TD.HK01 on relative heart weight, blood pressure parameters | ||||
| R. heart weight (mg/g) | 3.0910 ± 0.2326 | 4.3880 ± 0.2653*** | 4.5717 ± 0.4622***Δ | 4.4635 ± 0.4790***Δ |
| Systole BP (mmHg) | 200.88 ± 37.51 | 250.64 ± 43.27* | 253.75 ± 38.25* | 245.50 ± 44.90* |
| Diastole BP (mmHg) | 77.75 ± 2.76 | 75.82 ± 5.34 | 78.08 ± 1.93 | 76.58 ± 2.23 |
| Heart rate (p/m) | 374.13 ± 39.26 | 361.55 ± 99.66 | 410.08 ± 37.21 | 410.67 ± 59.93 |
| Effect of TD.HK01 on cardiac injury markers | ||||
| Troponin T (ng/mL) | 10.46 ± 3.38 | 4302.73 ± 1229.54*** | 2012.31 ± 670.28***ΔΔΔ | 1669.28 ± 432.28***ΔΔΔ |
| CK (IU/L) | 1675.24 ± 275.11 | 2678.8 ± 547.03*** | 2592.55 ± 686.07**Δ | 2488.68 ±
718.54**ΔΔ |
| CK-MB (IU/L) | 504.2 ±78.92 | 578.33 ±99.53 | 541.13 ±138.72 | 573.33 ±160.36 |
| LDH (IU/L) | 352.63 ±
84.53 |
708.3 ±
151.62*** |
569.25 ±
130.57** |
529.7 ±
101.83** |
| AST (IU/L) | 117.5 ± 18.13 | 391.73 ± 98.25*** | 313.67 ± 92.04*** | 248.64 ± 60.69***Δ |
| ALT (IU/L) | 38.00 ± 8.62 | 115.27 ± 27.22*** | 134.92 ± 28.39*** | 132.73 ± 37.68*** |
| Effect of TD.HK01 on catalase and eNOS levels | ||||
| CAT (ng/mL) | 11.92 ± 3.73 | 8.35 ± 1.82* | 15.30 ± 4.92ΔΔ | 15.04 ± 4.04ΔΔ |
| eNOS (ng/mL) | 2.49 ± 0.82 | 2.42 ± 0.50 | 7.34 ± 2.08***ΔΔΔ | 3.83 ± 1.15*ΔΔ |
Values are mean ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001 for chance differences vs control group. Δ p < 0.05, ΔΔ p < 0.01, ΔΔΔ p < 0.001 for chance differences vs negative control group.
Figure 1. Effect of TD.HK01 on ECG
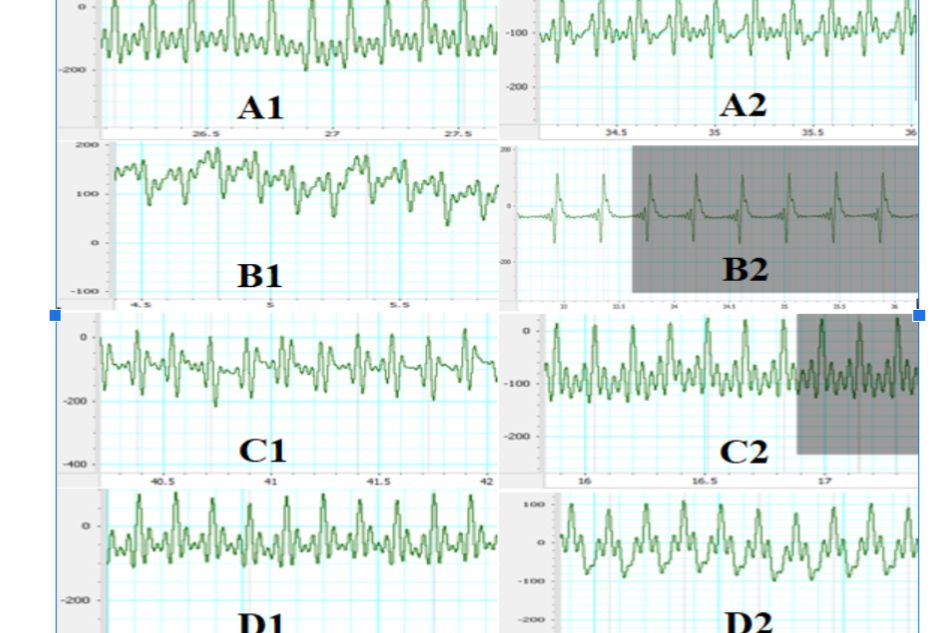
A. Normal control group, B. Negative control group (ISO 150 mg/kg, s.c.), C. Pretreatment TD.HK01 (0.31 g/kg) group, D. Pretreatment TD.HK01 (0.92 g/kg) group. 1. Before the study, 2. At the end of the study (48 hours after 1st ISO injection)
Figure 2. Histopathology of the hearts in ISO-induced MI rats
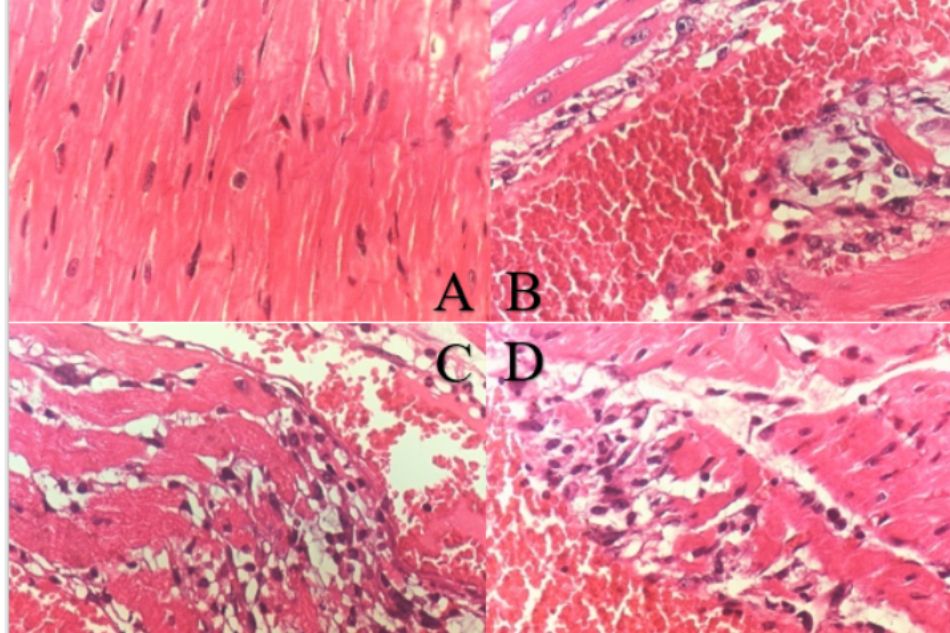 A. Normal control group, B. Negative control group (ISO 150 mg/kg, s.c.), C. Pre-treatment TD.HK01 (0.31 g/kg) group, D. Pre-treatment TD.HK01 (0.92 g/kg) group. Dash arrow indicates the vascular congestion; Solid arrow indicates leucocytes infiltration (LIF).
A. Normal control group, B. Negative control group (ISO 150 mg/kg, s.c.), C. Pre-treatment TD.HK01 (0.31 g/kg) group, D. Pre-treatment TD.HK01 (0.92 g/kg) group. Dash arrow indicates the vascular congestion; Solid arrow indicates leucocytes infiltration (LIF).


 한국어
한국어


